
Strategy and Statistics in Clinical Trials
698 kr
698 kr
On., 16 juli - ti., 22 juli
Sikker betaling
14 dagers åpent kjøp
Selges og leveres av
AdlibrisProduktbeskrivelse
Artikkel nr.
9891e0a4-43cc-4bf9-a758-05a98ac93d77
Strategy and Statistics in Clinical Trials
698 kr
698 kr
On., 16 juli - ti., 22 juli
Sikker betaling
14 dagers åpent kjøp
Selges og leveres av
AdlibrisLignende toppselgere

POP MART Labubu The Monsters Big Into Energy Series Figures Vinyl Plush Pendant Blind Box
699 kr

Øreputer for Bose QuietComfort - QC35/QC25/QC15/AE2 Hodetelefoner Svart
99 kr

POP MART Labubu The Monsters Exciting Macaron Vinyl Face Blind Box
699 kr

INF TYPE-C Dual SD/TF-kortleser for rask dataoverføring 0
89 kr
Tidligere laveste pris:
99 kr

Max Smekker
99 kr
Tidligere laveste pris:
164 kr

Luftrenseenhet - Renser / Saniterer luften - 20,000 mg/h
699 kr

Hundetrimmer / Potetrimmer - Trimmer for Poter
199 kr

Filter for MSPA oppblåsbare bassenger FD2089 4-pakning
289 kr

Malibu Fast Tanning Bronzing Butter with Beta Carotene 300ml
179 kr

NÖRDIC Lightning Kortläsare SD UHS-I
240 kr
Anbefalinger til dig

INF Etterfilter til Dyson V11 / V15 akselstøvsuger 3-pakning
229 kr

Afnan Supremacy Collector's Edition Eau De Parfum 100 ml (man)
781 kr

INF Støydempende og lydisolerende ørepropper med krok
114 kr
Tidligere laveste pris:
143 kr

INF Sovemaske 100 % silke 2-pack
164 kr
Tidligere laveste pris:
215 kr

INF Øreputer for Bose QC35 I/II, QC25, QC15, QC 2 AE 2, AE 2i, AE 2w, SoundTrue, SoundLink
99 kr
Tidligere laveste pris:
132 kr

RCA til HDMI Converter 1080p - Adapter
139 kr

INF Løkke for 22 mm klokkerem i 10-pakning Sort
69 kr
Tidligere laveste pris:
78 kr

INF Spiralreflekterende fugleskremsel 10-pakning Sølv 30 cm
119 kr
Tidligere laveste pris:
143 kr

INF Tilbehør til Roborock Q5 Pro+/Q8 Max+(Plus) robotstøvsuger
329 kr
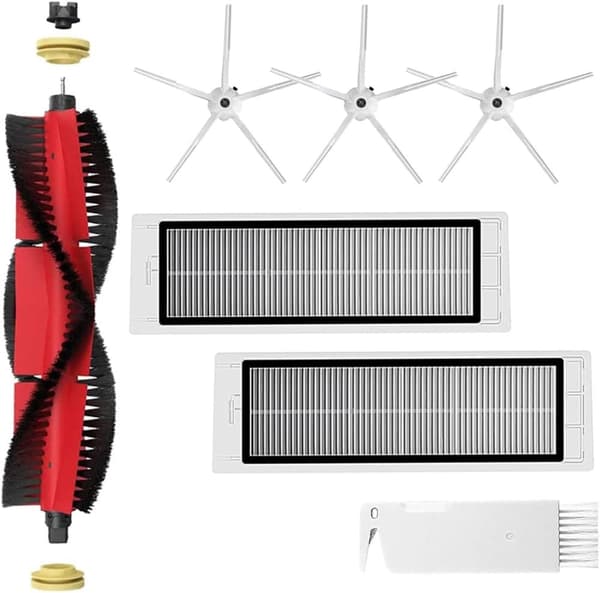
INF Tilbehør til Roborock S5/S6 modeller 7 deler
149 kr
Tidligere laveste pris:
228 kr