
Therapeutic Monoclonal Antibodies: From Lot Release to Stability Testing
1 579 kr
1 579 kr
På lager
Fr., 3 jan. - to., 9 jan.
Sikker betaling
Åpent kjøp til og med 7/1-25
Selges og leveres av
Adlibris
Produktbeskrivelse
Therapeutic Monoclonal Antibodies: From Lot Release to Stability Testing is a highly topical resource on a subject of interest for scientists and researchers working on monoclonal antibodies' characterization, release testing, stability testing and similarity assessments of monoclonal antibodies in the biopharmaceutical industry. Monoclonal antibodies (mAbs) are large, extremely complex and dynamic biomolecules, so it can be challenging to develop well-characterized therapeutic antibodies that meet regulatory expectations that are also in-line with commercialized standards for different drug markets. Lot release testing and understanding the stability of the mAb over a period of time, and in different environmental conditions, is an indispensable aspect of mAb physicochemical characterization. This book covers the process, including extensive analysis that starts with quantifying the purity attribute to glycan profiling and identifying the mAb primary structure. The book has a primary purpose of focusing on both Lot release testing and stability testing of monoclonal antibodies (subjects not covered in any great detail in other books).
Artikkel nr.
61e83379-c513-445b-9461-3bbf40bf8236
Therapeutic Monoclonal Antibodies: From Lot Release to Stability Testing
1 579 kr
1 579 kr
På lager
Fr., 3 jan. - to., 9 jan.
Sikker betaling
Åpent kjøp til og med 7/1-25
Selges og leveres av
Adlibris
Lignende toppselgere

Generic
Øreputer for Bose QuietComfort - QC35/QC25/QC15/AE2 Hodetelefoner Svart
99 kr
4,5(699)
tirsdag, 7 jan.

INF
Tilbehør Roborock S6/S60/S65/S5 Max/T6, 20 deler
289 kr
Tidligere laveste pris:
383 kr
2,9(20)
fredag, 3 jan.

Price Point
Universallader for Garmin klokker Svart
89 kr
4,2(148)
mandag, 6 jan.

Mobil o Teknik
PS4-kontroller DoubleShock Wireless
349 kr
onsdag, 8 jan.

INF
INF Sklisikker beskyttelse / brodder for sko med 18 stålpigger (L)
216 kr
4,3(20)
fredag, 3 jan.
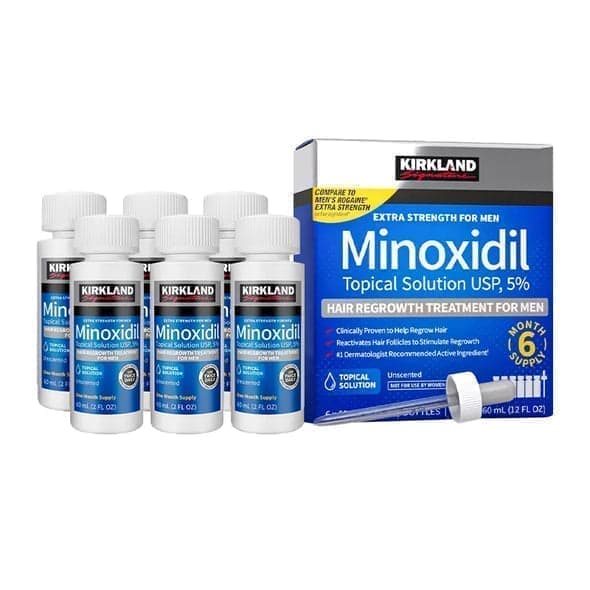
KIRKLAND
6x60ml = 360ml - Kirkland Extra Strength 5% Men Hair Regrowth 60ml Hair Loss
1 290 kr
5,0(4)
mandag, 6 jan.

INF
INF Overgang PS2 til HDMI m/ 3,5 mm lydutgang for HDTV-/HDMI-skjerm
123 kr
Tidligere laveste pris:
155 kr
4,0(23)
mandag, 6 jan.

Odin
Titan Silikon Til Tredemølle 100ml
149 kr
Tidligere laveste pris:
349 kr
5,0(4)
mandag, 6 jan.

Generic
2-Pak - PS4 Kontroll DoubleShock for Playstation 4 - Trådløs
549 kr
5,0(2)
tirsdag, 7 jan.

Vitu
Astronaut Night Light / Galaxy Lampe med fjernkontroll - Nepula Starry Sky Projector
299 kr
Tidligere laveste pris:
449 kr
4,1(34)
torsdag, 2 jan.
Anbefalinger til dig

Toppik
Toppik - 27,5g - Dark Brown - Mørkebrun
285 kr
Tidligere laveste pris:
379 kr
4,4(10)
mandag, 6 jan.

Generic
Knivsett for Barn 6-deler - Barnevennlige kniver
199 kr
4,4(17)
tirsdag, 7 jan.

Bengans
Jethro Tull - The Jethro Tull Christmas Album - Fresh Snow At Christmas (4CD+BD Boxset) (CD)
666 kr
5,0(1)
mandag, 13 jan.
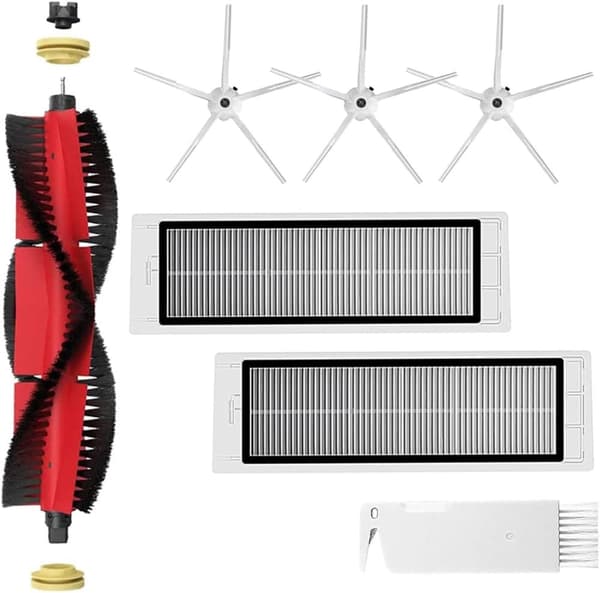
INF
INF Tilbehør til Roborock S5/S6 modeller 7 deler
181 kr
Tidligere laveste pris:
221 kr
4,2(48)
fredag, 3 jan.
Avatar - Limited Steelbook (4K Ultra HD + Blu-ray)
119 kr
Tidligere laveste pris:
149 kr
5,0(1)
mandag, 6 jan.

Azzaro
Azzaro Azzaro The Most Wanted Parfum edp 100ml
1 147 kr
4,7(6)
torsdag, 2 jan.

INF
INF Reparasjonssett for Nintendo Switch, 25 deler
173 kr
Tidligere laveste pris:
194 kr
4,6(33)
mandag, 6 jan.

Generic
2-Pak - iPhone Lader Adapter+Kabel 20W USB-C Hurtiglader
199 kr
3,7(500)
tirsdag, 7 jan.

B2x
Bambus kjøkkenutstyr sett med 7 stk
279 kr
5,0(1)
fredag, 17 jan.

Cozzy
Cozzy telys, 3D flamme, hvit, 8 stk inkl. fjernkontroll
189 kr
4,3(6)
torsdag, 2 jan.