
Nonclinical Development of Biologics, Vaccines and Specialty Biologics
1 807 kr
1 807 kr
På lager
To., 6 feb. - on., 12 feb.
Sikker betaling
14 dagers åpent kjøp
Selges og leveres av
Adlibris
Produktbeskrivelse
Nonclinical Development of Biologics, Vaccines and Specialty Biologics, Second Edition, is a complete reference devoted to the nonclinical safety assessment of novel biopharmaceuticals, vaccines, cell and gene therapies, and oncology therapeutics. Updated and revised, the new edition compares and contrasts these types of biologics with one another and with small molecule drugs, while incorporating the most current and essential international regulatory guidelines. This book discusses the different types of biologics, as well as early characterization strategies, principles of study design, nonclinical pharmacokinetics and pharmacodynamics, nonclinical assays, and regulatory guidelines. A coedited book with chapters authored by leading experts in the field, this comprehensive reference provides critical insights to all researchers involved in early through late-stage biologics.
Artikkel nr.
82d103c3-dd48-5ab8-a41b-d11e796ad79e
Nonclinical Development of Biologics, Vaccines and Specialty Biologics
1 807 kr
1 807 kr
På lager
To., 6 feb. - on., 12 feb.
Sikker betaling
14 dagers åpent kjøp
Selges og leveres av
Adlibris
Lignende toppselgere

Generic
Øreputer for Bose QuietComfort - QC35/QC25/QC15/AE2 Hodetelefoner Svart
99 kr
4,5(715)
mandag, 27 jan.

Megabilligt
Galaxy Lamp - Nebula Star -projektoren med fjernkontroll
499 kr
Tidligere laveste pris:
909 kr
3,8(28)
mandag, 27 jan.

INF
Universallader for Garmin klokker
90 kr
4,3(262)
fredag, 24 jan.

CherrysC
Squid Game 2 Gonggi & Case Korean
129 kr
fredag, 31 jan.

Brädspel.se
Spill Kluster (SE/FI/NO/DK)
321 kr
4,9(8)
onsdag, 29 jan.

Maaleo
Dobbeltsidige tusjer/penner - 168 stk. MAALEO 24101
399 kr
3,0(2)
torsdag, 6 feb.

INF
INF Sklisikker beskyttelse / brodder for sko med 18 stålpigger (L)
259 kr
Tidligere laveste pris:
291 kr
4,4(22)
torsdag, 23 jan.

Odin
Titan Silikon Til Tredemølle 100ml
149 kr
Tidligere laveste pris:
349 kr
4,3(6)
fredag, 24 jan.

Generic
2-Pak - PS4 Kontroll DoubleShock for Playstation 4 - Trådløs
549 kr
4,1(9)
mandag, 27 jan.
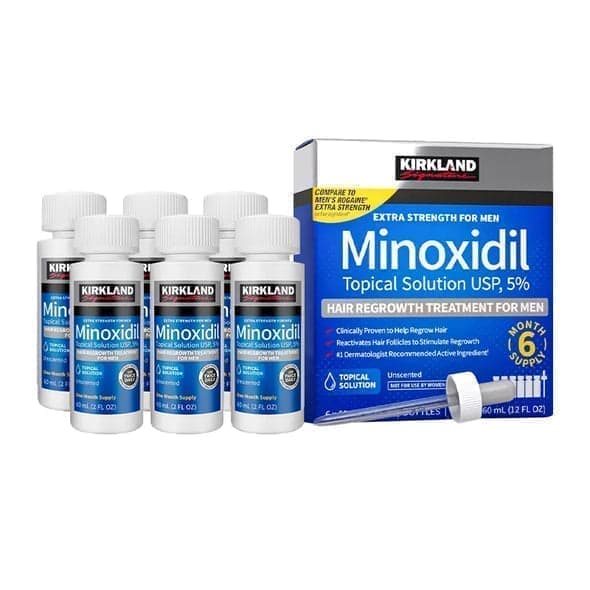
KIRKLAND
6x60ml = 360ml - Kirkland Extra Strength 5% Men Hair Regrowth 60ml Hair Loss
1 290 kr
5,0(5)
fredag, 24 jan.
Anbefalinger til dig

Generic
2-Pak - iPhone Lader Adapter+Kabel 20W USB-C Hurtiglader
199 kr
3,7(503)
mandag, 27 jan.

PlayStation 5 Slim Digital Edition (PS5)
5 789 kr
4,4(38)
fredag, 24 jan.

Mobil o Teknik
Elektrisk babyneglefil - fra nyfødt til småbarn
199 kr
4,0(5)
tirsdag, 28 jan.

B2X
Sett med skjærebrett i bambus i metallstativ med unik design
409 kr
Tidligere laveste pris:
439 kr
4,3(10)
torsdag, 6 feb.

Generic
RCA til HDMI Converter 1080p - Adapter
129 kr
4,4(15)
mandag, 27 jan.

INF
INF Ankelstropp for styrketrening med kabelmaskiner 2 Pack
147 kr
Tidligere laveste pris:
149 kr
4,4(5)
fredag, 24 jan.

Iconsign
Lash Lift Kit - Permanent Lash Curling Kit - Iconsign Original
179 kr
3,6(17)
onsdag, 22 jan.

INF
INF Stylus Pen kompatibel med iPad 2018-2023-serien HvitiPad
196 kr
Tidligere laveste pris:
268 kr
4,1(43)
fredag, 24 jan.

INF
INF Sett for bartending med dobbel shaker 750ml i rustfritt stål, 10 redskaper
272 kr
Tidligere laveste pris:
397 kr
4,5(57)
torsdag, 23 jan.
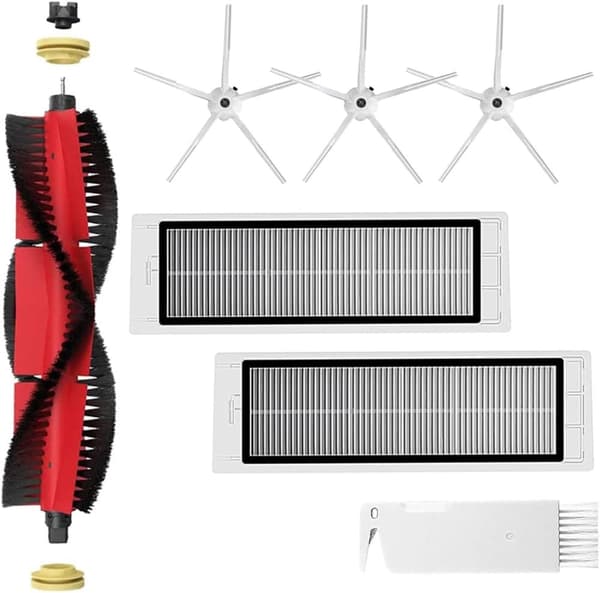
INF
INF Tilbehør til Roborock S5/S6 modeller 7 deler
219 kr
4,2(49)
torsdag, 23 jan.