
Clinical Trial Project Management
1 755 kr
1 755 kr
Tidligere laveste pris:
1 763 kr
På lager
On., 29 jan. - ti., 4 feb.
Sikker betaling
14 dagers åpent kjøp
Selges og leveres av
Adlibris
Produktbeskrivelse
Clinical Trial Project Management provides a detailed overview of how to conduct clinical trials in an international context. The process of conducting clinical studies across nations is based on a set of regulatory regimes developed by respective regulatory agencies. The book focuses on clinical study protocol approval processes, Ethics Committee approval processes, clinical study feasibilities, site selection, site initiation, site monitoring, database lock, sit close-out, clinical data processing and management, SAE reporting and compensation, randomization procedure, pharmacovigilance, statistical tools, BA/BE studies, and clinical study report writing. The book comprehensively covers the entire clinical trial process of conductance. In addition, the author also incorporates the clinical trial approval processes of USFDA, EMA, and JAPAN for conducting clinical trials.
Artikkel nr.
4a4af648-44d7-587f-8b5f-dc152f86aec1
Clinical Trial Project Management
1 755 kr
1 755 kr
Tidligere laveste pris:
1 763 kr
På lager
On., 29 jan. - ti., 4 feb.
Sikker betaling
14 dagers åpent kjøp
Selges og leveres av
Adlibris
Lignende toppselgere

Generic
Øreputer for Bose QuietComfort - QC35/QC25/QC15/AE2 Hodetelefoner Svart
99 kr
4,5(715)
mandag, 27 jan.

CherrysC
Squid Game 2 Gonggi & Case Korean
129 kr
fredag, 31 jan.

INF
Universallader for Garmin klokker
90 kr
4,3(262)
fredag, 24 jan.

Megabilligt
Galaxy Lamp - Nebula Star -projektoren med fjernkontroll
499 kr
Tidligere laveste pris:
909 kr
3,8(28)
mandag, 27 jan.

Odin
Titan Silikon Til Tredemølle 100ml
149 kr
Tidligere laveste pris:
349 kr
4,3(6)
fredag, 24 jan.

Maaleo
Dobbeltsidige tusjer/penner - 168 stk. MAALEO 24101
399 kr
3,0(2)
torsdag, 6 feb.
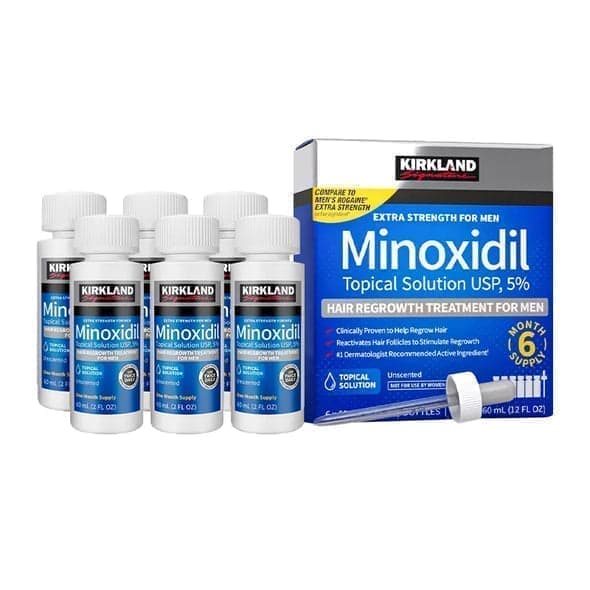
KIRKLAND
6x60ml = 360ml - Kirkland Extra Strength 5% Men Hair Regrowth 60ml Hair Loss
1 290 kr
5,0(5)
fredag, 24 jan.

Generic
2-Pak - PS4 Kontroll DoubleShock for Playstation 4 - Trådløs
549 kr
4,1(9)
mandag, 27 jan.

INF
INF Sklisikker beskyttelse / brodder for sko med 18 stålpigger (L)
259 kr
Tidligere laveste pris:
291 kr
4,4(22)
torsdag, 23 jan.

Generic
2-Pak - iPhone Lader Adapter+Kabel 20W USB-C Hurtiglader
199 kr
3,7(503)
mandag, 27 jan.
Anbefalinger til dig
INF
INF Hudormfjerner i rustfritt stål 3 deler Sølv
106 kr
5,0(4)
fredag, 24 jan.

INF
INF Stylus Pen kompatibel med iPad 2018-2023-serien HvitiPad
196 kr
Tidligere laveste pris:
268 kr
4,1(43)
fredag, 24 jan.

PlayStation 5 Slim Digital Edition (PS5)
5 789 kr
4,4(38)
fredag, 24 jan.

B2X
Sett med skjærebrett i bambus i metallstativ med unik design
409 kr
Tidligere laveste pris:
439 kr
4,3(10)
torsdag, 6 feb.

INF
INF Reparasjonssett for Nintendo Switch, 25 deler
189 kr
4,6(33)
fredag, 24 jan.

INF
INF Ankelstropp for styrketrening med kabelmaskiner 2 Pack
147 kr
Tidligere laveste pris:
149 kr
4,4(5)
fredag, 24 jan.

Nördic
NÖRDIC 192kHz DAC Digital til Analog Converter SPDIF Toslink til Analog Stereo RCA L/R
350 kr
4,1(14)
fredag, 24 jan.

INF
INF Sett for bartending med dobbel shaker 750ml i rustfritt stål, 10 redskaper
272 kr
Tidligere laveste pris:
397 kr
4,5(57)
torsdag, 23 jan.
INF
INF Tilbehør til Roborock S5/S6 modeller 7 deler
219 kr
4,2(49)
torsdag, 23 jan.

Mobil o Teknik
Elektrisk babyneglefil - fra nyfødt til småbarn
199 kr
4,0(5)
tirsdag, 28 jan.